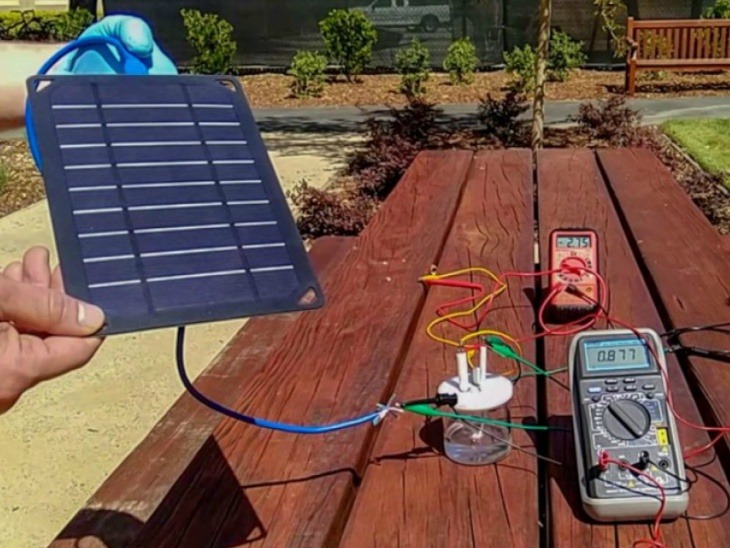
Researchers at Stanford University have developed a way to make hydrogen fuel by sea water. According to the report printed in the National Academy of Sciences, oxygen and hydrogen can be separated by using sea water using electricity. This fuel can be used to provide electricity to the cities and to give energy to cars.
By separating oxygen and hydrogen from water, the hydrogen fuel that can be used in place of conventional fuel is already being made. But in this work pure water is needed, which is very precious in itself. Researchers say that it was not possible to use hydrogen fuel to illuminate the entire city and to power the cars, as it would require very high amount of pure water. Stanford University researcher H Die, Yun Kuang and Michael Kenny said that getting pure water as much as our needs in California is also a bit difficult. But in the new research, the sea salt water will be converted into fuel.
Hydrogen fuel use is also good for nature because it does not emit carbon dioxide. Burning hydrogen fuel only emits water, which does not help in increasing the problems of climate change. Whereas conventional fuels emit carbon dioxide which is harmful to nature.
Google TV is great out of the box, but these apps can take your viewing…
The world of streaming in India is about to look a whole lot different! Get…
Realme is gearing up to launch its latest flagship, the Realme GT 7 Pro, on…
Xiaomi has confirmed that the Redmi A4 5G will be launched in India on November…
The Realme 14 series is gearing up for its India launch, now set for January…
Oppo’s much-anticipated Reno 13 series is likely to be unveiled around November 25. This series…