Researchers at Stanford University have developed a way to make hydrogen fuel by sea water. According to the report printed in the National Academy of Sciences, oxygen and hydrogen can be separated by using sea water using electricity. This fuel can be used to provide electricity to the cities and to give energy to cars.
Spending avoidance of salt water
By separating oxygen and hydrogen from water, the hydrogen fuel that can be used in place of conventional fuel is already being made. But in this work pure water is needed, which is very precious in itself. Researchers say that it was not possible to use hydrogen fuel to illuminate the entire city and to power the cars, as it would require very high amount of pure water. Stanford University researcher H Die, Yun Kuang and Michael Kenny said that getting pure water as much as our needs in California is also a bit difficult. But in the new research, the sea salt water will be converted into fuel.
Better options for nature
Hydrogen fuel use is also good for nature because it does not emit carbon dioxide. Burning hydrogen fuel only emits water, which does not help in increasing the problems of climate change. Whereas conventional fuels emit carbon dioxide which is harmful to nature.
Increasing productivity from 12 hours to 1000 hours by laying the negative ion layer
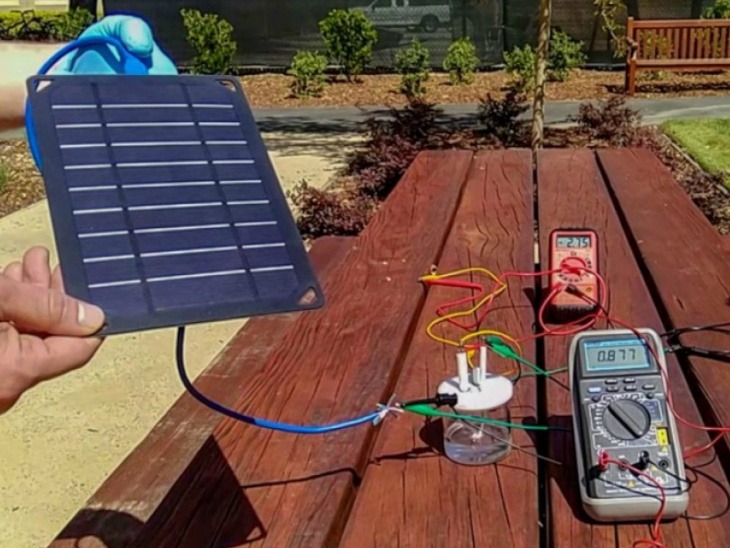
- The process of breaking the water into hydrogen and oxygen with the help of electricity is called electrolysis. This is an easy and old way. One power source is added to two electrodes placed in the water. When power is turned on, the hydrogen emits from the negative end (cathode) of the electrode, while the oxygen comes out of the positive end (anode) of the electrode.
- In this process, using sea water can damage the positive end of the chloride electrode with negative ions present in the water. Stanford’s researchers raised the negative ion layer on the positive end (anode) of the electrode to solve this problem, which causes the process of degrading the anode to a great extent.
- Without the negative ion layer, the anode can work in saltwater for only 12 hours. But after laying the layer it can be used for one thousand hours.
- The team has also developed a solar energy-powered system, which can be used to replace hydrogen fuel by using solar energy instead of electricity.
- Hydrogen derived from this process can be used for fuel. The oxygen emitting from it is breathable, hence its use can also be used to make oxygen tanks. This system can also simplify the dynamics of divers.


